1
/
of
13
Zepteruae
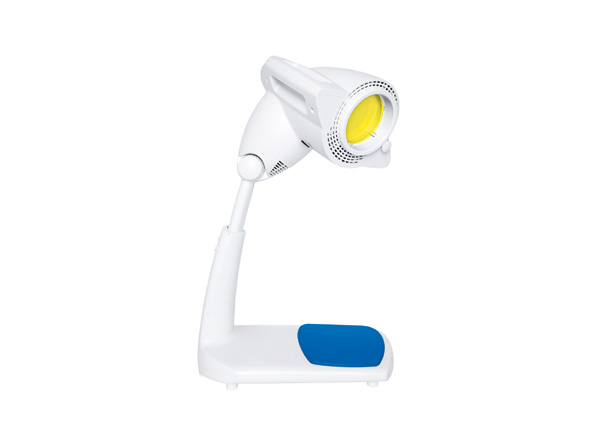
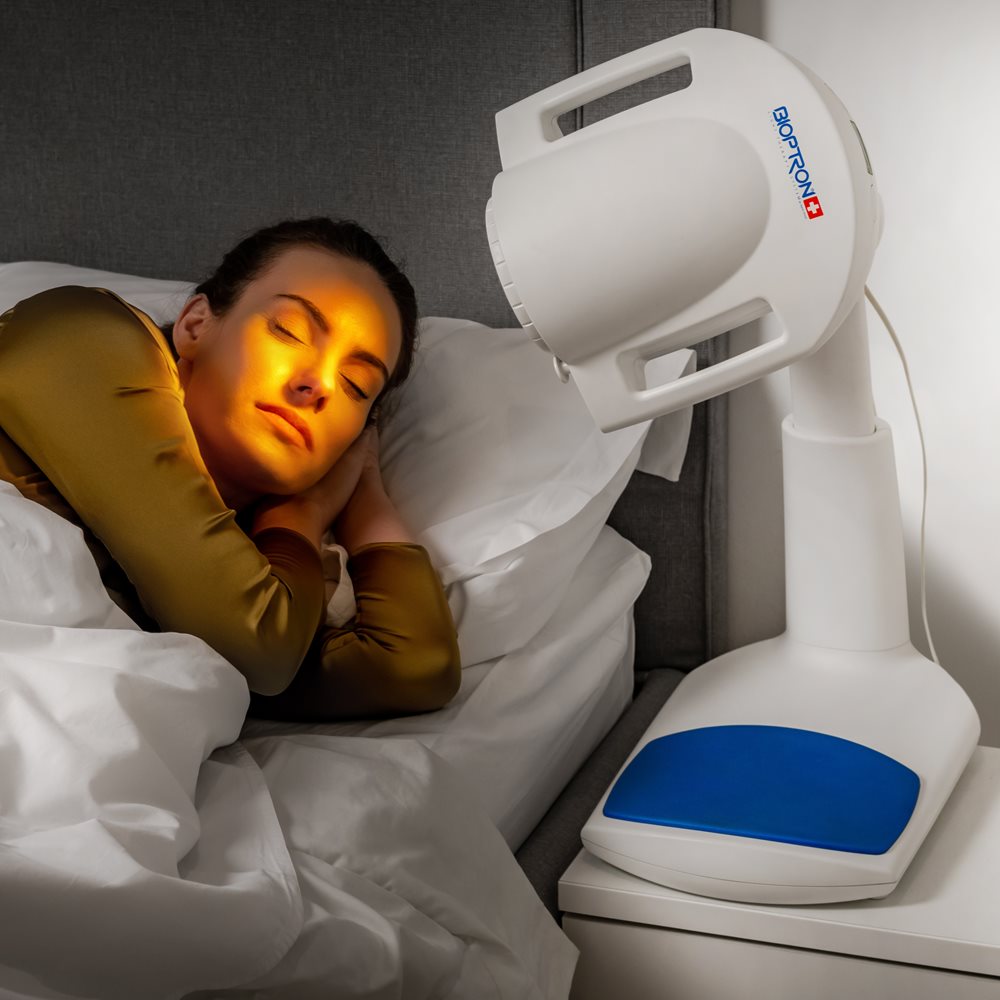
BIOPTRON PRO1 set with table stand PAG-990
BIOPTRON PRO1 set with table stand PAG-990
Regular price
Dhs. 11,200.00 AED
Regular price
Sale price
Dhs. 11,200.00 AED
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
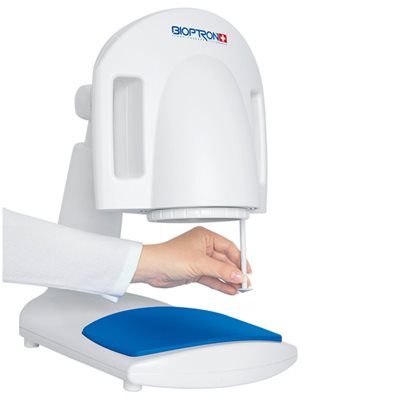
Our light technology is based on Nobel Prize winning technology, and is fully certified for non-invasive, accelerated healing and prevention of a wide range of conditions including: acne, skin wounds, sports injuries, anti-aging, arthritic and low back pain. Just 5 to 10 minutes of treatment with BIOPTRON twice a day is all that is needed for accelerated healing. BIOPTRON Pro 1 has a filter diameter of 11cm.
Tech Data
Item code: PAG-990
Product name: BIOPTRON PRO1 set with table stand and hyperlight optics
Gross weight [KG]: 6.05
Net weight [KG]: 4.55
Weight with stand: 6.05kg gross / 4.55kg net
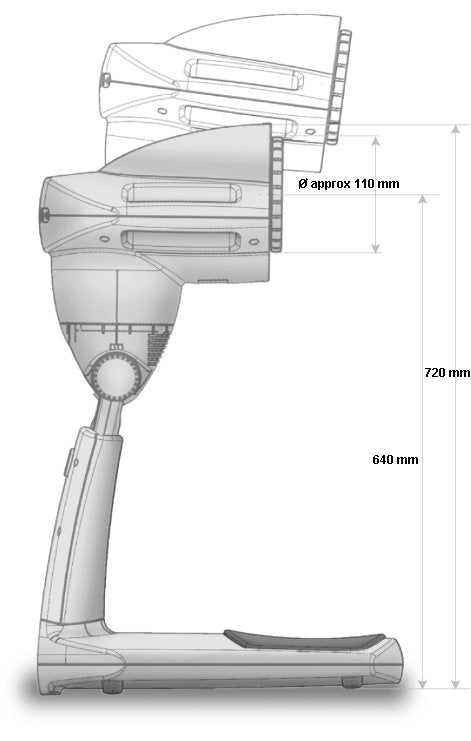
Dimensions in box: 40 x 30 x 49 cm
Producer: BIOPTRON AG - Sihleggstrasse 23, CH-8832 Wollerau - Switzerland
Made in: Switzerland
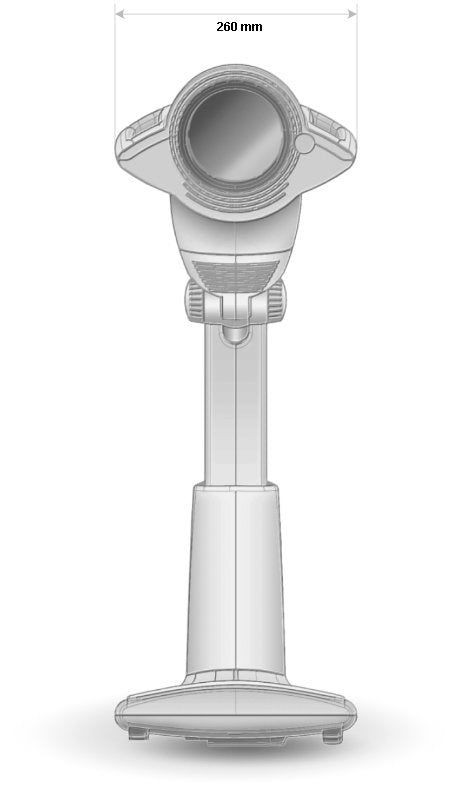
Filter/glass diameter: ap. 11 cm
Color: White
Voltage: Power supply 100-240 V~, 50/60 Hz
Power consumption: 75 W
Rated power of halogen: 50 W
Power cord: detachable
Light energy per minute: an av. of 2.4 J/cm2
Protection against overheating: YES
Power density: on av. of 40 mW/cm²
Safety class: IIa IP20
Setting therapy time: YES
Working temperature: when used 10°C to + 30°C; for storage 0°C to + 40°C
Wavelength: 350 - 3400 nm
Degree of polarization: >95% (590 - 1550 nm)
Composition: BIOPTRON PRO1 device with basic filter * Table stand * Power cord (detechable) * Protective band on babies eyes * Protective cover * Protective bag * User manual * Warranty
Display: YES- digital
Warranty: 5 years
Certifications/Declaration:
* Declaration of Conformity with the Directive 93/42 / EEC issued by the producer. *Confirmation of application device in the Office for Registration Products, Devices and Biocidal Products * CE conformity for electrical equipment. * Certificate for Quality Assurance (EN ISO 13485) * Certificate for the Quality Assurance System (Directive 93/42 / EEC) issued by the FDA * DEKRA Certificate for quality control EN ISO 13485:2012 + AC:2012 * DEKRA Certificate for devices complying with Annex II, Section 3 of Directive 93/42 / EEC - (Notified Body ID 0124) * Declaration of conformity from DEKRA (European notified body) for all products issued in 2013 (07.21.2013)
Share